Featured photo courtesy of Daniel R. Blume.
The past two posts in this series introduced and explained the concepts of electromagnetic radiation, and the electromagnetic spectrum. To further the discussion of why I believe sunlight is crucial to health, I will focus on how light is produced in the sun, and the specific spectrum that the sun emits (i.e. the parts of the electromagnetic spectrum hitting the earth from the sun).
Sunlight is, well, the light that is emitted by the sun (I’m so smart). What is causing this light to be emitted? What is the source of this light? Is it fire? As a lot of you will already know to a certain degree, the source of all the energy coming out of the sun (and pretty much all stars) is nuclear fusion. Essentially, it is the smashing together of atoms to produce bigger atoms, which releases an inordinate amount of energy as a by-product. This is different than fire (i.e. combustion) in that fusion fuses atoms together to form larger atoms, while combustion simply rearranges atoms within molecules.
One of the most common and basic particles in the universe is the proton. The proton has an electric charge of positive (+). There are also particles called electrons that have an electric charge of negative (-), but they aren’t involved in the process of nuclear fusion. Protons are often combined with neutrons, which have no electric charge, but are about the same size and mass as the proton.
Positive charges attract negative charges and vice-versa via the electromagnetic force (“opposites attract”). Positive charges repel other positive charges, just like negative charges repel other negative charges (“like charges repel”). This is like those old experiments in elementary school where you rubbed your head against balloons (transferring negatively charged electrons to the balloon) and then you noticed that your balloon was repelling your buddy’s balloon, but the balloons would stick to the wall. The balloons repelling each other is explained by the electrons from your hair getting built up on the balloons giving them each a net negative charge (and repel…). The wall; however, was neutrally charged (no charge, or pretty close), but attracted the negatively charged balloon due to the charge difference.
The dominant nuclear fusion process occurring in the sun that produces the vast majority of light is called the proton-proton chain. In essence, protons combine together to form the larger helium atoms in a multi-step chain reaction. As mentioned above, protons are positively charged, so they repel each other by the electromagnetic force. To overcome the electromagnetic repulsion, the protons have to be traveling extremely fast. This happens when the immense gravity of the sun pulls together enough protons and compresses them so tightly that they get extremely energetic, and can overcome the electromagnetic repulsion that results from their respective positive charges. Once they get close enough, the weak nuclear force becomes much stronger than the electromagnetic repulsion and the two protons fuse together releasing gamma radiation in the process. Gamma radiation, if you’ll recall from my last post, is generally known as the highest energy category of electromagnetic radiation. Below you’ll see an illustration of one step in the proton-proton chain where a gamma ray is released. A proton fuses with deuterium (proton + neutron) to form Helium-3.

Once again, my stellar art skills are on display. Anyone want to help me out with this, or recommend a better drawing program than Paintbrush? Keep in mind, I rock a MacBook.
Although the main process of energy production in the sun produces gamma rays, the Earth does not get nailed with too many of these. The fusion process takes place in the centre of the sun, where the pressure from gravity is the absolute strongest. Since the gamma rays have to travel from the centre of the sun to the surface to be emitted into space, the gamma rays get absorbed and re-emitted by all the particles between the centre and the surface of the sun. Each time this process occurs, the rays have slightly less energy, so by the time the energy reaches the surface, for the most part, the energy of the radiation has been ‘knocked-down’ to categories of electromagnetic radiation that are much lower in energy. Typically, the energy is emitted as a mix of infrared, visible, and ultraviolet radiation. This is shown in the figure below:
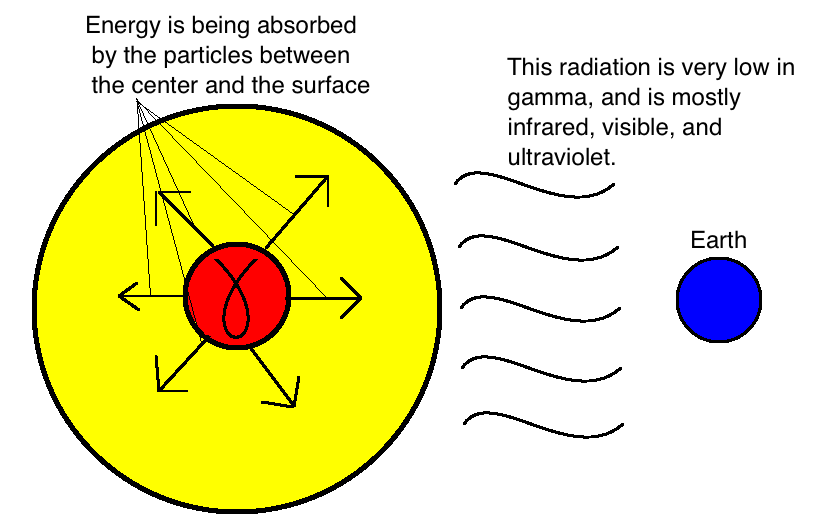
The ‘furnace’ of the sun puts out annihilating gamma radiation, but all the stuff in between the centre and the surface absorbs a lot of the energy.
Each star, including our sun, has it’s own special ‘signature’ of radiation that it emits. This is based on many factors including the mass of the star, the composition of the star, and the age of the star. The sun (‘our’ star) is pretty much middle-of-the-road in many respects. It’s a very common type of star, known as a main-sequence star. A discussion of all the different types of stars and their life cycles is beyond the scope of this post, but it should be clear that each star emits a unique radiation spectrum that changes throughout the life cycle of the star, but is relatively constant for much of the star’s life. So, as seen in the above figure, our sun emits mostly in the infrared, visible, and ultraviolet categories of electromagnetic radiation. This can be visualized in the figure below (hail Wikipedia!):

Yellow is what hits the Earth, and red is what hits the surface of the Earth. The atmosphere absorbs a bunch of it before it gets to the surface, so there is a lower irradiance across all wavelengths.
To explain the above figure, “Spectral Irradiance” (the vertical axis) just means, “how much of this particular wavelength of energy hits a certain area”. The horizontal axis is the wavelength (in nanometers, a meter divided by 1 billion, so really small) of the electromagnetic energy that the sun emits. At the top of the figure, there are labels for the dotted lines that represent where the divisions are for the infrared, visible, and ultraviolet (UV) categories. The higher a particular wavelength is on the vertical axis, the more of that wavelength is emitted by the sun.
The yellow part of the figure is the radiation emitted by the sun, and the red part of the picture is what is left of the radiation after Earth’s atmosphere has absorbed some of it. Focus on the yellow part of the picture for now. You’ll notice that the highest spectral irradiance is the visible band (literally the wavelength that is the highest on the vertical axis). Although the wavelength with the highest irradiance is in the visible category, there is actually more total infrared if the irradiance from all the infrared wavelengths are added up. Ultraviolet only makes a small portion of the entire signature. In terms of the entire categories, it actually works out to about 49% infrared energy, 40% visible energy, and about 10% ultraviolet energy. And about 1% ‘everything else’ (other categories of electromagnetic radiation).
10% ultraviolet. This, from my previous post, is the type of electromagnetic energy that has enough ‘pop’ to start breaking molecules apart. It’s the reason that sunlight is considered the dominant cause of skin cancer.
The red portion of the figure, as mentioned above, shows what is left of the sun’s radiation after the atmosphere absorbs a chunk of it. The relative size of the red UV spectrum as compared to the yellow UV spectrum shows that most of the ultraviolet radiation gets absorbed by the atmosphere. The ozone layer is largely responsible for this absorption.
That will be the topic of the next post! I will be discussing the interaction of the sun’s radiation with our atmosphere, and how that affects the radiation that actually hits our bodies. You may be surprised at how little ultraviolet we actually get exposed to, especially in modern times.
Until then, I bid you adieu. As always, if you have any questions or comments, please do not hesitate to leave a comment on the blog.
- G
