Featured image courtesy of Miriam.
My last post discussed the nature of light, and that our current understanding is that light can be thought of as an electromagnetic wave carrying varying amounts of energy expressed by different wavelengths, or frequencies.
The differences in the wavelengths/frequencies creates the electromagnetic spectrum.
The electromagnetic spectrum takes us all the way from the radio waves that we tune into while we’re driving in the car, to the light our eyes can actually see, to high-energy waves that can tear apart atoms. I found a dope illustration of this on Wikipedia:
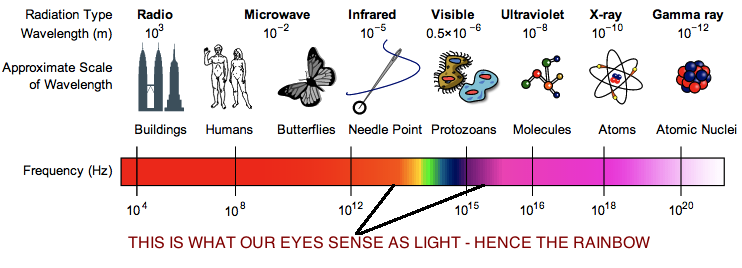
It may surprise you that radio waves and microwaves are electromagnetic radiation just the same as visible light. You’ve probably heard of infrared, ultraviolet, x-ray, and maybe even gamma radiation. There is only a very small part of the spectrum that our eyes can detect and that we consider ‘light’. In reality, the window that is visible light is actually much smaller than what is depicted above, and is actually a tiny sliver of the entire spectrum. The other categories of electromagnetic radiation interact with the environment and us differently, but they’re fundamentally the same phenomenon as visible light. Light is broadly categorized into about eight different types of radiation, as shown above. There’s a fair bit of overlap, but the distinctions are still useful.
Here’s a brief rundown of each of the main types, in order of lowest-energy to highest-energy:
Radio – These are the friendly guys we tune into to get all the hottest tracks. We have learned to embed information in these low-energy waves by either frequency modulation (FM) or amplitude modulation (AM). Television signals use radio waves as well. These guys aren’t dangerous since the strength of the signals we use for communication are weak (very few waves, relatively speaking), and interact with matter very modestly.
Microwave – The type of radiation used, you guessed it, in your microwave oven! These guys are energetic enough to be absorbed by certain molecules and get them vibrating (i.e., increase their energy). Water is one of those molecules. So, all the water in food gets hot in your microwave oven, and that’s what transmits the heat to the rest of the food. Cell phones transmit information on the low-energy side of the microwave band, but at very low signal strengths. Other than being able to heat certain substances if the signal strength is high enough, microwave radiation is quite safe.
Infra-red – Okay, now we’re having fun. Infra-red is quite simply HEAT. And not wussy heat like microwaves. This type of radiation has enough energy to get pretty much everything vibrating and rotating and getting HOT. Hot objects, like the element on your stove, radiate a lot of infra-red energy, and even human bodies radiate at about the middle of the infrared range. ‘Infra-red’ goggles are thus ‘heat goggles’, which means that the goggles pick up infra-red energy, and translate that energy to visible light, so that whoever is wearing the goggles can now ‘see’ heat. Works great when you are man-hunting at night. Ever heard of infrared lamps? That’s a heat lamp. Simple. ‘Infra’, means inferior. ‘Red’ means red. So infrared is inferior to red. Makes sense when we look at the next category.
Visible light – Literally, this is the stuff that we see with our eyes. This category is energetic enough to be absorbed and re-emitted by electrons. This is the radiation responsible for photosynthesis. Every colour has a specific frequency/wavelength. The lowest energy (lowest frequency) being red, while the highest energy (highest frequency) being blue/violet. This explains the colours in the rainbow (ROYGBIV – red, orange, yellow, green, blue, indigo, violet). The way water vapour scatters light is wavelength-dependent, so higher energy light gets scattered at a slightly larger angle then lower energy light, which spreads out the colours and creates a rainbow. ‘Infrared’, explained above, quite literally means ‘inferior to the colour red in terms of energy’. The next category, ultraviolet, means ‘superior to violet in terms of energy’.
Ultraviolet – Now we’re getting pretty high-energy. As implied in the name, this category of radiation is higher in energy than violet light, and we can no longer see this radiation with our eyes. The energy inherent in ultraviolet is where things can start to cause serious ‘damage’ and why radiation from the sun gets a bad rap. On the low-energy side of the UV spectrum, if an electron absorbs this type of radiation (creating an ion), it can get stripped off its host atom, which changes the behaviour of the atom. Increasing the energy within this range, and molecules are torn apart (pulling atoms away from each other). For the most part, the entire UV range is split up into the subcategories UV-A, UV-B, and UV-C. UV-A is lowest in energy, and UV-C is highest. These subcategories have different interactions with matter, as will be explained in the coming posts.
X-rays – This category of radiation has frequencies higher than UV, and like UV can literally tear molecules apart. However, on the higher end of the X-ray range, the energy is so high that the radiation doesn’t efficiently interact with the soft tissues of our bodies (it still does to a degree, and when it does it’s fierce). Our bones however, contain metals (calcium, magnesium) that do efficiently absorb X-rays. This is why X-rays are used for medical imaging. You shoot a bunch of X-rays through a person, and only the bones can stop them, so we get a negative image on the detector screen behind the person of his/her bones. A bit of radiation damage in the soft tissues does happen, but the X-rays are finely tuned to minimize this, and the benefits (hopefully) outweigh the costs. That’s why there are limits on the amount X-rays you can get in a year. Don’t mess around.
Gamma – There is quite a bit of overlap in terms of frequency range with X-rays, with the only real distinction being that gamma rays originate from the nucleus of an atom. So, this is what powers nuclear power plants (along with other wavelengths), and what (mostly) comes blasting out of a nuclear bomb. This stuff is super intense, and pretty much tears apart everything. Small and controlled doses are used in medical imaging and cancer therapy where, similar to X-rays, the benefits of the radiation dose are hoped to outweigh the damage incurred.
When it comes to electromagnetic radiation, it’s same shit, different pile (actually, it’s one continuous pile). The next post will take a look at how electromagnetic energy is produced in the sun, and what the Earth is getting showered with. After that we’ll be taking a look at how the electromagnetic radiation from the sun interacts with our bodies (skin, eyes) and why I think we shouldn’t be avoiding it entirely (hint: there is a goldilocks zone – i.e. not too much, not too little, just right… and it’s more sun than you might think).
Stay tuned for the next one! Please, if you have any questions, post a comment. I will answer you.
– G

This post is very interesting but it took me a long time to find it
in google. I found it on 13 spot, you should focus on quality backlinks building, it will help you to rank
to google top 10. And i know how to help you, just type in google – k2 seo tips
I have learn some good stuff here. Certainly worth bookmarking for revisiting. I surprise how much attempt you set to create any such excellent informative website.
beats fake
[url=http://www.religionandnature.com/beats/beats-fake.html]beats fake[/url]
Why thank you! I’m glad you learned something. I’m planning on finishing the Sunlight Series this spring. Still designing the website! Cheers!