Featured photo courtesy of Tamara.
Before a detailed discussion on the effects of sunlight on the body can begin, I believe the reader should be oriented with the concept of ‘light’ itself, which is the intention of this post. I’m not going to explain optics,
but focus upon the question of the nature of light, and explain our current understanding of what it is. First up, light in any form has no mass. Second, light is, well, quite mysterious in that it can behave as a wave, or as a mass-less particle called a photon (it’s called wave-particle duality). All particles can behave as waves as well (physics nerds – deBroglie!), but it’s far more obvious and measurable with light. Keep that in mind as we continue.
The wave-view of light, which is what we’ll look at for now, is best thought of as a combination of oscillating electric and magnetic fields travelling through space. An electric field is a region of space, produced by charged particles, that applies a force on charged particles (like the proton or the electron). A magnetic field, simply explained, is a field produced by moving charged particles that applies a force on charged particles that are moving. Physicists in the 19th century (most notably Michael Faraday and James Clerk Maxwell, among others) figured out that electric and magnetic forces were inherently the same phenomenon, and thus these forces were combined into the electromagnetic force.
Due to this nature, light is also called electromagnetic radiation. It can be pictured like so:
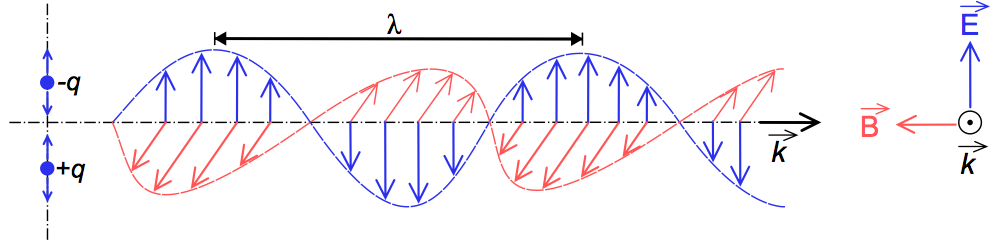
I could give a bit more of a theoretical explanation of the concept, but it would be beyond what we need for the conversation at hand (leave me a comment and I’ll get rambly for you!). The ‘wavelength’ of the electromagnetic radiation, mentioned in the caption above, determines how much energy the light is carrying. Essentially, the shorter the wavelength, the more energy is contained in the light. The longer the wavelength, the less energy. Since light moves at the speed of light(~300,000 km/s, which is crazy fast), a shorter wavelength means that the electromagnetic field is oscillating faster (i.e. going up and down faster as in the picture above). If the electromagnetic field oscillates slower, that increases the wavelength. This can be thought of as ‘it takes longer to go up and down, so it travels further during that time, which increases the length of the wave’. You dig?
The rate that the electromagnetic field changes is expressed with the frequency of the light. The frequency specifies ‘how many times the electromagnetic field bounces up and down in a specified time frame’. At a rate of going all the way down then back up (called a cycle) once per second, this is one Hertz (Hz). It’s named after Weinrich Hertz, the scientist who figured a bunch of this stuff out in the late 1800’s.
SO – with that all in mind, electromagnetic radiation, which is light, can be summed up like this:
HIGH ENERGY = HIGH FREQUENCY = SHORT WAVELENGTH
LOW ENERGY = LOW FREQUENCY = LONG WAVELENGTH
The entire spectrum of long electromagnetic waves to short electromagnetic waves is called, well, the electromagnetic spectrum, and you’ll soon find out that what we consider ‘light’, is only a small portion of the entire spectrum, with some other familiar names in the mix.
That will be the next post! The electromagnetic spectrum, which will explain how different wavelength ranges are broken up into subcategories that interact with matter in different ways.
Please, as always, leave a comment if you have any questions.
-G
