When I meet other health/fitness/nutrition minded folk and mention that I follow a mostly Primal/Paleo lifestyle, a lot of the following discussion centres around defending my decision to limit grains in my diet, or how much meat I eat. What usually doesn’t come up, or isn’t focused on much, is the restriction or even elimination of industrial seed oils such as soybean, corn, cottonseed, and canola oil in favour of healthy animal fats, coconut oil, olive oil, oily fish, and small amounts of nuts. This, however, is one big freaking deal! Industrial seed oils are cheap and highly prevalent, and unless you’re paying attention, you’re getting more of those fats than you think.
If you want to skip all the sciency details and just get to the results, there is a table at the end of this post that summarizes everything nicely. For all those who want to know why, read on!
My view on good fats and bad fats is in opposition to the standard conventional view. The reasons will be explained below, but I think you’ll be convinced that eliminating or limiting most industrial seed oils/vegetable oils is one of the most important nutritional moves you can make regarding your health. There will be some science involved, but I’ll try to make it fun, I promise! There are good fats, and there are bad fats. To see the difference, first we must understand fats. Let’s do it.
What is fat? What are fats?
Fat, in the context of your diet, is what’s known as a macronutrient. “Macro” means BIG (opposite of micro, which means small). Macronutrients form the structural and energy giving components of our diet, and include fats, carbohydrates, and proteins. You get a relatively large amount of fat in your diet (measured in grams), unlike a micronutrient where you get a relatively small amount (like say, zinc or other minerals or vitamins, measure in milligrams, or 0.001 grams). Basically, the amount of fat you consume on a daily basis can be seen with the naked eye – you can hold it in your hand and feel it, unlike a micronutrient where it’s a tiny amount.

Sometimes the term “oil” is used as well, but when it comes to food, oils and fats are almost the same thing. Typically, “oils” refer to fats that are liquid at room temperature, while “fat” is for fats that are solid at room temperature. Chemically, there are differences that have an impact on the physical properties, which will be explained in more detail below. Oil in this context is not to be confused with petrochemical oil, which is used to produce fuels like gasoline, or materials like plastics. Rather, we will be talking about edible oils that come from plants and animals. Fats and oils do not mix with water, and are slippery to the touch. Hence forth in this article I will use the terms fats and oils interchangeably. With more detail below, you’ll understand why. Sometimes the plural will be used (fats), and sometimes the singular (fat), but it’s the same thing.
For the most part, fat is used by the body as a source of energy. Our cells can burn fats of various types to supply it’s metabolism with energy. Certain fats are also chemical starting points for important compounds our bodies make. I know some of you might be thinking that “fat is bad” or “fat makes you fat”, but here’s the thing: that is only true under certain contexts, while under normal and natural circumstances, using fat for fuel is perfectly healthy, and often preferable to using glucose (sugar), the main alternative. In fact, swapping out sugar for fat can actually reduce the incidence of cardiovascular disease!
Fats in the diet can come from many sources. Animal fats like butter, bacon grease, lard, duck fat, beef fat, and others, tend to be solid at room temperature. Plant sources tend to be liquids at room temperature, with examples like olive oil, avocado oil, nut oils, and seed oils. I can’t recommend the seed oils (reasons below!), but the rest are all great stuff. The notable exception to this rule-of-thumb is coconut oil, which breaks the trend by being solid at room temperature.
To understand fats further, we’re going to rock some chemistry! I’ve made this easy to visualize by adding illustrations from hired artists on Fiverr.com. Let’s get into it!
The chemistry of fats explained
Fats are made up two types of components: 1. A “glycerol” backbone, and 2. up to three “fatty acids”, attached to glycerol. Both the fatty acids and glycerol can be metabolized for energy, but in this article, we’ll be focusing on the fatty acids. So, without further ado, let’s take a look at a fat molecule:
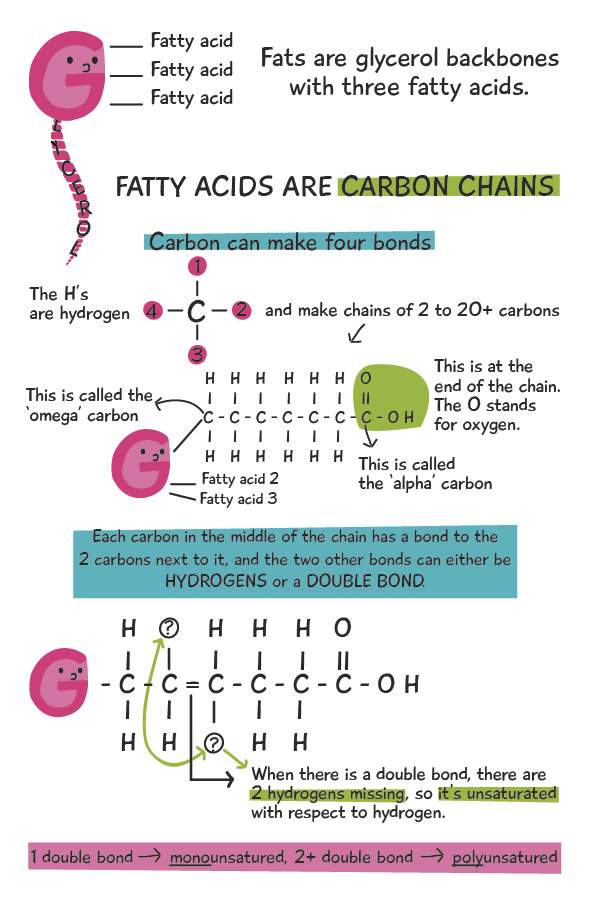
When it comes to fatty acids within fat molecules, the degree of unsaturation (with respect to hydrogen) makes a big difference in the physical and chemical qualities of the fats. Although the diagram above shows the fatty acids as straight, in reality the double bond causes a ‘kink’ or a bend in the chain that doesn’t allow the fatty acids to pack closely together, like in a solid. Due to this effect, saturated fatty acids (no double bonds) are solid at room temperature, while mono- and polyunsaturated fatty acids are generally liquids at room temperature. This explains the physical properties of the fatty acids. The chemical properties will be explained below. Henceforth, saturated fatty acids will be SFAs, and mono and polyunsaturated fatty acids will be MUFAs and PUFAs, or simply UFAs to describe both of them.
Fat molecules usually have three fatty acids, but can also have two or one. When there are three it’s known as a triglyceride, and when there are two or one, the terms diglyceride and monoglyceride are used, respectively. What’s known as a type of “fat” (e.g. lard, butter, olive oil, etc.) is a collection of mono, di, and triglycerides that have a range of different fatty acids that make up the fatty acid profile of the fat/oil. Fats with a higher concentration of SFAs as compared to UFAs are more solid at room temperature, and likewise, fats with a higher concentration of UFAs as compared to SFAs are more liquid at room temperature. The image below illustrates this concept. The fats with more SFAs (red), are more solid at room temperature (lard, butter, coconut, etc.), while the oils with more UFAs (white and green) are more liquid (peanut, soybean, olive, etc).
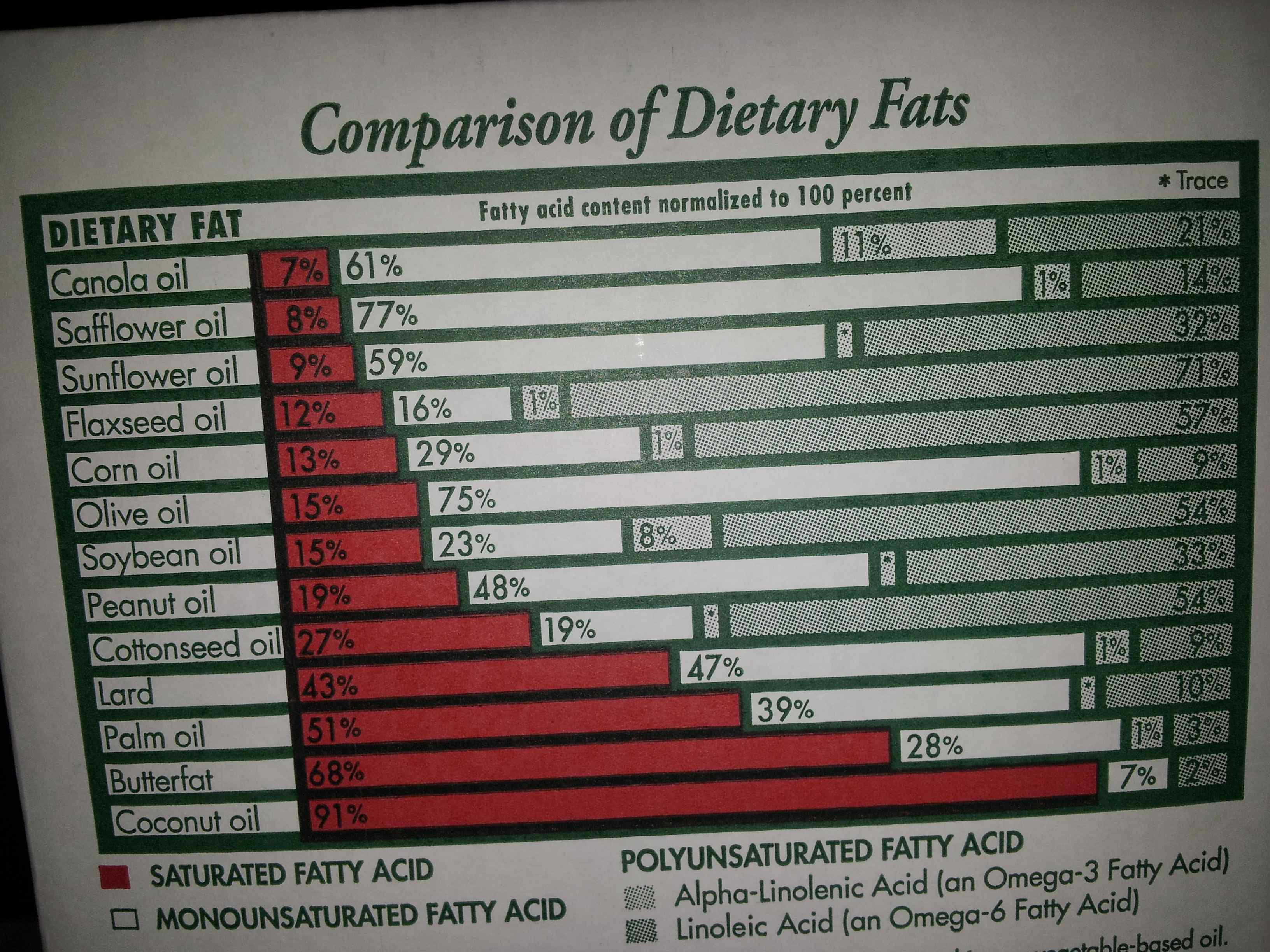
Fatty Acid Chemical Properties
OK, so that explains why some fats are liquids and some are solids, but why else does it matter? Well, as mentioned above, the different fatty acids have different chemical properties. This means that the different fatty acids will undergo different chemical reactions depending on the conditions they’re in.
How UFAs go Rancid! Ack!
Specifically, the double bonds in the UFAs produce surprising effects. The second bond (of the double), is weaker than the first bond. The weaker second bond is very prone to cleavage, which means that second bond gets chemically cut in half. When the second bond is broken, it becomes a highly-reactive free radical. These free radicals react with oxygen and produce several nasties in the form of aldehydes, ketones, and other toxic substances linked to degenerative illnesses such as advanced aging, neurological disease, heart disease, and cancer. This process is known as rancidification, producing rancid oils. It is aka oxidation, or degration. The figure below illustrates this process.
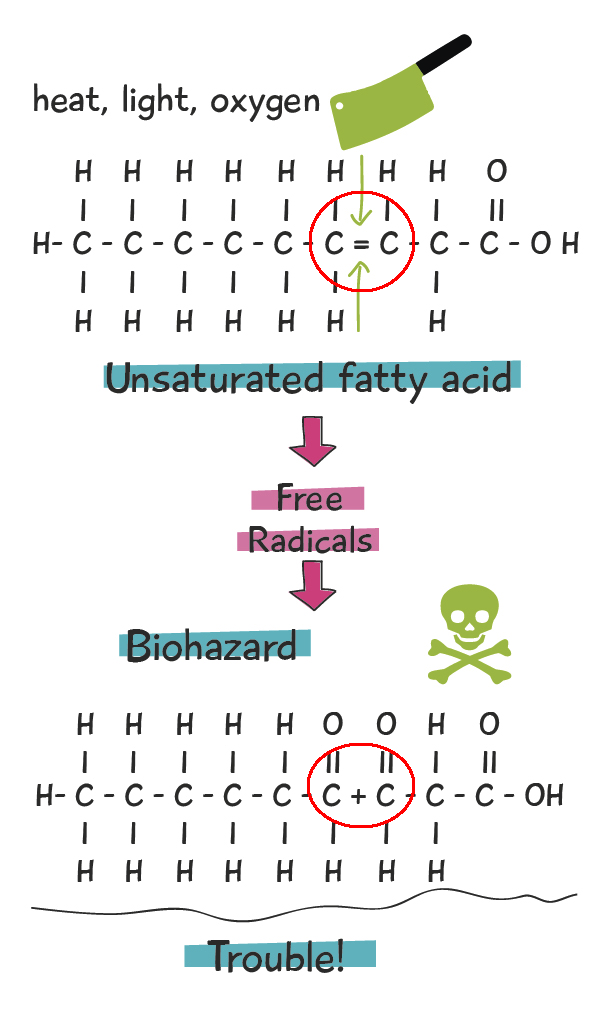
All it takes is some exposure to oxygen, heat, or light, and this toxic process gets underway! Remember though, it’s the double bonds in UFAs, and especially PUFAs that are making these reactions so easy. SFAs and MUFAs hold up much better to thermal stresses (heat) than PUFAs. SFAs are the most stable, with MUFAs not too far behind, and PUFAs being by far the most prone to rancidification.
In Defense of Saturated Fat
But wait, aren’t SFAs bad for you? Aren’t these the fats that raise cholesterol and cause heart disease? Shouldn’t we be limiting SFAs to be healthy? I know that’s what the American Heart Association would tell you, but I’m here to tell you, backed by modern research, that SFAs are perfectly healthy, and actually a great source of fuel for your body. It’s the excessive and rancid PUFAs in processed vegetable oils (soybean, canola, corn, etc) that are unhealthy. All the crap about SFAs being bad (from butter, bacon, other animal fats, etc.) was complete garbage. All a big lie that the general public has been fed for decades to the point where it has become false gospel. This short clip from FatHead (documentary) explains it best:
PUFA: Essential for life, but rancid and out of whack!
When it comes to PUFAs, so far I have been painting a pretty bad picture. What I’m trying to do is convince you that excessive and rancid PUFAs are bad, not that any amount of PUFAs are harmful.
In fact, two main types of PUFAs, Omega-3 and Omega-6, are called essential fatty acids because, well, you need them from your diet to survive! You will literally die without them. Our bodies incorporate these fatty acids right into our cellular membranes (the “skin” around a cell), and also use these fatty acids as a chemical starting point to make all sorts of things that are essential to life. In this sense, these PUFAs aren’t always used as a source of energy, but more often as a structural or chemical component of your body.
As alluded to above, the two main types of PUFAs in our diet are omega-3, and omega-6. If you’ll recall from the first graphic above, the omega carbon is the one attached to the glycerol in the fat molecule. Now, if we start counting carbons along the chain, starting at the omega carbon, if the first double bond is at the 3rd carbon, it’s an omega-3. If the first double bond is at the 6th carbon, it’s omega-6. Simple, right?
Now, there are distinct plant and animal versions of the omega-3’s and omega-6’s. The plant forms need to be converted to the animal version for us to actually use them, or better yet, we can get them in the animal form straight from our diets, and things go much smoother.
Vegetable oils, seeds, and nuts are high in plant-based omega-6, and are generally low in omega-3. The big exceptions here are chia and flax, that are very high in plant-based omega-3, and relatively low in plant-based omega-6. For animal-based omega-6, we’re looking at pork, chicken, and some beef (you get some of the plant form from these animals as well). The classic prescription for animal-form omega-3? Fish and all seafood! That’s the best way to get it. Grass-fed ruminants (cattle, sheep, goats) also have some animal-based omega-3. Here are the fancy chemical names:
Plant forms:
Omega-3: alpha-linolenic acid (ALA)
Omega-6: linoleic acid (LA)
Animal forms:
Omega-3: eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA)
Omega-6: arichidonic acid (AA)
In general, omega-3’s are anti-inflammatory, and omega-6’s are pro-inflammatory. Ideally, we’d have a ratio of omega-6 to omega-3 of about 1:1, which would keep their effects in natural balance. Problem is, if you look above at the fatty acid profiles, the vast majority of the PUFAs from the vegetable oils are omega-6! Consuming vast amounts of these oils is a new phenomenon, and in the modern world, omega-6:omega-3 ratios of 10:1 are the norm, and can even go as high as 30:1! We are getting way too much omega-6, and not enough omega-3! It’s out of whack! Soybean, corn, cottonseed, and canola oil being used in place of the more traditional fats like lard, butter, olive oil, and fatty fish are largely responsible for this, and the results have been that we are experiencing more inflammation than ever, with drastic health consequences.
OK, so we need these fatty acids to survive, but we only need small amounts, and hopefully with a balanced omega-6:omega-3 ratio. We already know that they degrade very easily if they are exposed to light, heat, or oxygen, so we shouldn’t heat them up. This all combines to say:
Industrial Seed Oils: DO NOT CONSUME
By industrial seed oils, I mean soybean, corn, sunflower seed, cottonseed, and to a lesser extent, canola oil, amongst a few others. These oils are colloquially referred to as vegetable oils, although there are no vegetables involved. To be clear, I do not mean olive oil, avocado oil, or macadamia oil. These oils actually come from fruits (except macadamia, but it’s an exception), and are easily extracted and mostly MUFAs, and are chock full of antioxidants that protect against rancidification (although you still have to be a bit careful). It’s the high-PUFA industrial oils that concern me.
Now, eating some whole-food corn or a handful of sunflower seeds is not a big deal. The problem occurs when we consume large amounts of the chemically extracted and refined oil from these foods on a regular basis. The source of the problem is three-fold:
1. Oil from these ‘foods’ (cotton? come on) is hard to extract, so often lots of heat and chemical solvents are used.
Chemical solvents like hexane are used to coax out the oil from ground up seeds. I mean, there’s not much oil to begin with, so there’s a lot of work to get it out. Let’s use corn oil as an example.Think about it – have you ever considered corn oily? It’s not very oily. Generally speaking, the oil is about 2.8% of the weight of the corn. There is a tiny amount of oil in there, and it’s tough to separate it out, so chemical solvents become necessary, and traces of these solvents are left in the final product. Lots of heat is also used in this process, which we know is a problem because:
2. Industrial seed oils/vegetable oils are very high in polyunsaturated fatty acids.
Take another look at the fatty acid profiles above. These oils are replete with PUFAs. That means that during the high-heat oil extraction process, the PUFAs are getting degraded and becoming rancid. Yikes! This stuff is toxic. Believe it.
There are expensive and time-consuming oil extraction methods that do not require chemicals and heat, but these are rare, and even when they are used, the longer the oil sits around being exposed to light and oxygen, the more rancid it becomes. Even if it was somehow protected, these oils are still not ideal because:
3. The vast majority of the excessive PUFAs in seed/vegetable oils are omega-6.
We know that we’re getting way too much omega-6 already (these oils being part of the problem), so why add fuel to the fire? Even if the fancy low-heat, chemical-free extraction methods are used, and the oil is somehow safe from oxidation, you don’t want to be getting too much of these! The big two exceptions to the omega-6 issue with seed oils are flax seed oil, and chia seed oil. These oils, while having ample omega-3 and low omega-6, are mostly the plant-based form of omega-3, so they’re not that valuable anyway (unless you’re vegan and refuse to eat fish), and are still prone to rancidification.
In the end, there’s just not much reason to consume seed/vegetable oils, and plenty of reasons to avoid them. I personally hope this message will spread, and our food industry will change to protect our health, and not just the food industry’s profits.
Trans Fats: Hydrogenation Gone Wrong
With all the seed oils prevalent in the food industry, food companies started thinking that they could change the physical and chemical qualities of the high-PUFA oils to be more solid at room temperature and resistant to oxidation. Remember – the liquid properties of these oils and the susceptibility to rancidification is due to the large amount of double bonds in the fatty acids, which are unsaturated with respect to hydrogen. So – they figured, “Why don’t we add some hydrogen back? Let’s force these oils to undergo hydrogenation”.
OK – at first glance, maybe this is a good idea! We’ll turn those PUFAs into MUFAs and SFAs, and we’ll get a better fat in return that can stand up to heat better. Oh, if only it were that simple.
Here’s what ends up happening: there are two arrangements that the double bond can conform to, 1. a ‘cis’ arrangement, and 2. a ‘trans’ arrangement. In nature, the vast majority of double bonds conform to the cis arrangement, while there are relatively few double bonds that are in the trans configuration. When high PUFA oils undergo hydrogenation, some of the fatty acids undergo the problematic partial hydrogenation, which means they do not become completely saturated. When this happens, a lot of the double bonds (~25-45%) convert to the trans arrangement. These specific ‘trans fats’ are consistently linked to an increase in cardiovascular disease. These are toxic, mutant fats that we should not consume.
That being said, there are some natural and healthy trans fatty acids that are present in the fats from grass-fed ruminant species (cattle, sheep, goats), in relatively small amounts. These fats are not to be feared, and are quite healthy. It’s the trans fats resulting from the partial hydrogenation of high PUFA vegetable oils that are bad news.
Smoke Point vs. Fatty Acid Stability
Typically, the property called “smoke point” is used to determine which fats/oils are suitable for which temperature to cook at. This concept, however, doesn’t take into account that a lot of the rancidification of the PUFAs happens without really visible “smoke” per se. If you look at this Wikipedia list of oil smoke points, a lot of the seed/vegetable oils I have been referring to as evil actually have some of the highest smoke points. This is because smoke point does not take into account the stability of the fatty acids. A lot of the time, the degree of refinement of the oil has the biggest effect on the smoke point.
If there’s a lot of plant/animal matter left in the oil (like virgin olive oil or butter), then the plant/animal matter will start to burn and you’ll get smoke. If the oils are refined, then it’s just pure fat with nothing else, and the smoke point goes up significantly.
The best example of this is butter vs. ghee. Butter still has milk solids and proteins kicking around, and according to Wikipedia, butter starts to burn at between 121-149 C. Ghee is clarified butter, which means all the milk solids and proteins are removed, and it’s just the pure fat. Due to this refinement process, ghee enjoys a much higher smoke point of 252 C!
Another example using vegetable oil – unrefined canola oil smokes at 107 C, while refined canola oil smokes at 204 C!
So, while smoke point is important for flavours in your food (don’t want it to taste burned), it doesn’t indicate whether the fatty acids are stable under high heat.
In general – stick to mostly saturated fats for high heat cooking, and use more unsaturated fats light cooking and salad dressings or as a topping. If you need to cook at extremely high temperatures for a long time (200+ C), go for a very refined, mostly saturated fat like refined coconut oil or ghee. Also ask yourself – why am I cooking at such a high temperature for such a long time?
Thank you so much if you’ve bared with me this long! We are almost done. Time to summarize!
How do I use this wonderful information?
All the info presented above can be summarized with the following table:
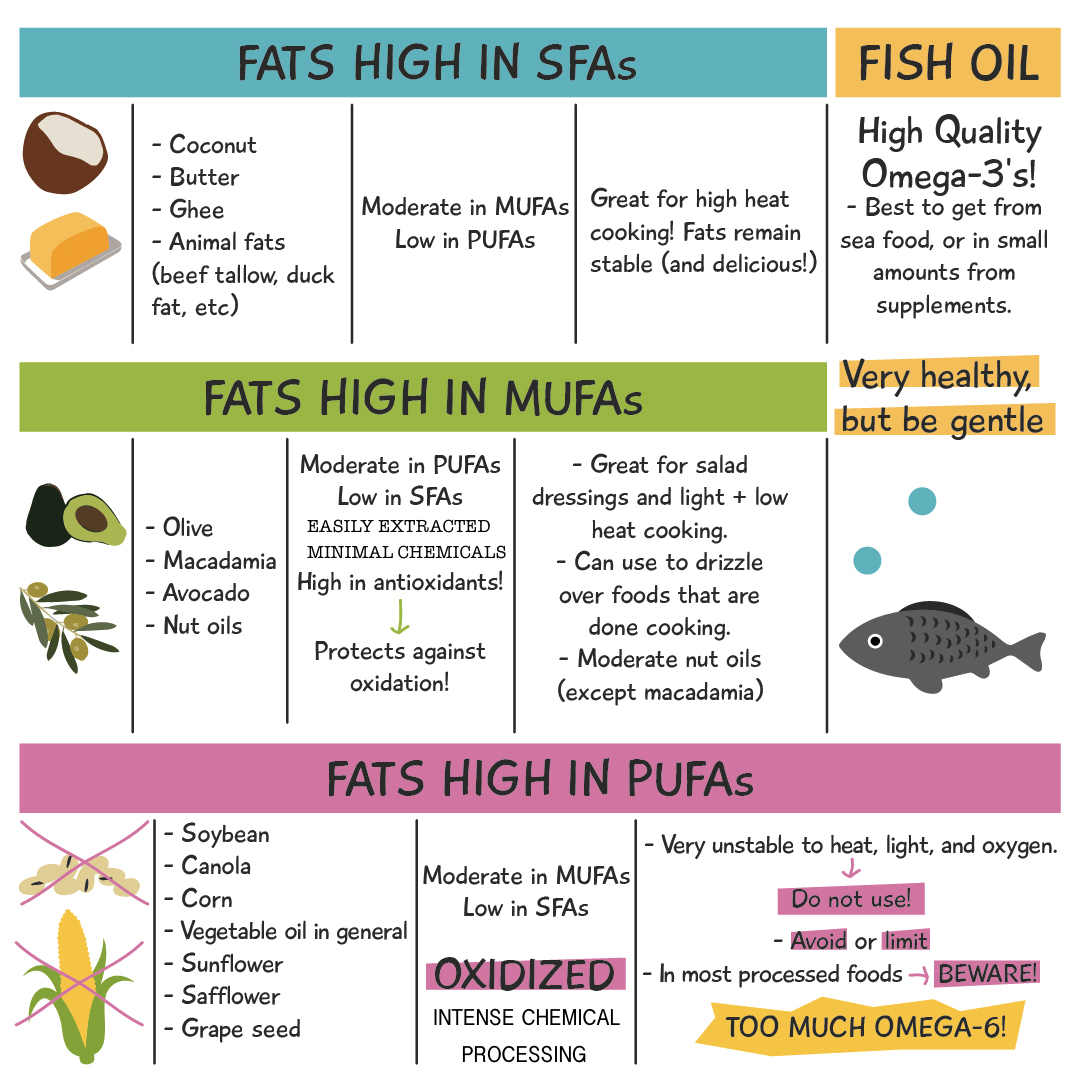
There you have it! I hope this has been educational for you, and that you can use this information to make better choices when it comes to the glorious fats in your diet.
Stay informed, stay smart, stay healthy!
If you have any questions, please ask away in the comments section, and I will answer.
Here’s to your health,
Graham
