With regards to energy, so far I have talked about why energy is awesome, gone over the basics of energy, and explored how the consumption of energy affects quality of life. With all that out of the way, I want to start taking a look at energy sources that are alternatives to fossil fuels (fossil fuels accounting for over 80% of current energy sources).
For this post, let’s take a look at a very polarizing energy source: nuclear energy. I was initially intending to cover nuclear energy in one post, but as I have now come to expect, I had to split it up into several posts. This post will be an over view of what nuclear energy is and the types of reactions that produce it. The next post will take a look at the various types of nuclear fuel cycles (in use today, and experimental), and the advantages and disadvantages of the different approaches.
To start off, let’s take a look at how much nuclear energy is used in the grand scheme of things. The figure below shows how much nuclear energy was being used in 2012 as part of the entire primary energy supply (not just electricity generation), as compared to 1973. There has been some growth! Nothing too substantial (we’re still at less that 5% of all energy supplied globally), but as oil prices go up and nuclear technology improves, that piece of the pie could grow considerably.

OK – but what is nuclear energy exactly?
What is Nuclear Energy?
Nuclear energy seems like a dangerous, unnatural energy source used to make bombs or to stack the bank account of Mr. Burns, but it’s really a completely natural source of energy that has been around for eons. In fact, as explained in my article How is Energy Produced in the Sun?, the energy released from the sun and all stars is nuclear, so nuclear energy has been warming and illuminating our planet for over four billion years.
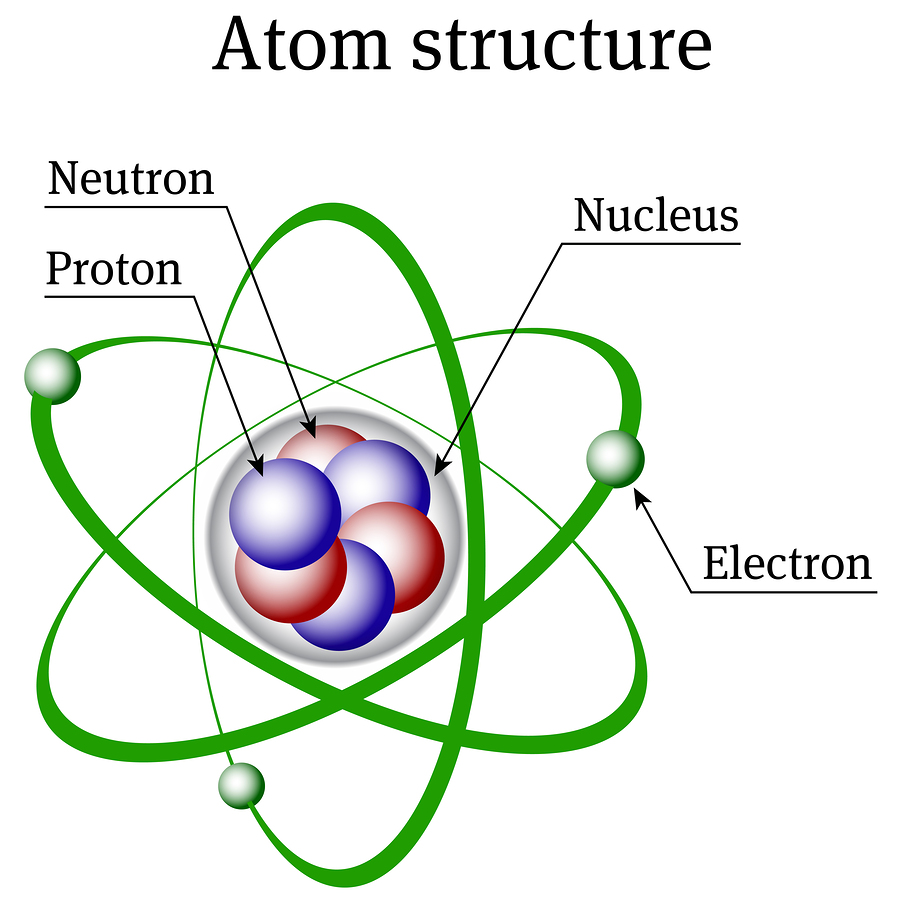
Nuclear energy has also been called “atomic energy”. This is because the atom, which is the building block of almost all the physical matter around us, has within it a nucleus, which is where 99%+ of all the mass of the atom is contained. The atom looks like the following figure, where the nucleus is composed of the protons and neutrons at the center.
The atomic number of an atom is determined by how many protons are in the nucleus. This atomic number determines which element on the periodic table the atom is. There can be any number of neutrons within a nucleus, and it won’t change which element the atom belongs to (different amounts of neutrons within a nucleus correspond to different isotopes of that same element). Electrons, as seen in the above diagram, exist in a cloud around the nucleus.
So, where does energy come into this? Well, as you may recall from high school science class, the protons and the electrons within an atom have an electric charge. The proton is positively charged, and the electron has an equal but opposite charge (negatively charged). In the world of electric charge, opposites attract, and like charges repel each other. But wait! All those protons stacked together in the nucleus are all positively charged, and should be repelling each other, right? It takes a huge amount of energy to keep all the protons together, and this my friends, is nuclear energy. The neutrons add extra nuclear forces (energy) without extra repulsion (they have no charge), so they help to stabilize the nucleus.
To make things slightly more complicated (as nature is apt to do), up until there are 26 protons in the nucleus, energy gets released as more protons are added (you chemists will note that this is the element iron). After 26, we start losing energy, BUT we can move in the opposite direction and remove protons/neutrons to release energy. It’s all about nucleus manipulation en masse.
That’s all fine and dandy, but all that energy is locked up in the nucleus. How do we unleash this energy and turn it into a form we can use?
Nuclear Reactions
It is through nuclear reactions (like in the sun) that we harness nuclear energy and use it for our own means. This is different from chemical reactions that are the source of energy from fossil fuels. As hinted at above, nuclear reactions come in two major categories: 1. fusion, where we combine two different nuclei together to make a bigger one, and 2. fission, where we take a big nucleus and split it up into smaller ones.
Here’s the really interesting part: Most people have heard of Einstein’s famous equation E = MC2, but few have used and understand it. E stands for energy, M stands for mass, and C2 stands for the speed of light squared (speed of light is 300,000 km/second). This equation relates mass to energy. Without getting too philosophical about it (we’ll save that for another time), Einstein discovered that mass and energy were the same thing, but in different forms. This means that it is very possible to convert mass to energy, and vice versa.
This is what happens in a nuclear reaction. If the products of a reaction has less mass than what you started with, the mass deficit is converted into pure energy (of various forms – heat, light, etc.). This can happen for both fusion and fission. Since the speed of light is a huge number, the speed of light squared is a ridiculously huge number. This equates to a tiny amount of mass converting to a massive amount of energy.
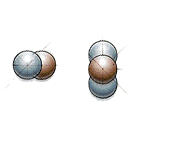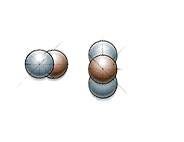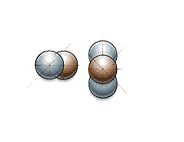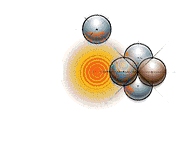
Here’s what fusion looks like:
And this is what fission looks like:

In practice, only nuclear fission is used to produce energy in nuclear reactors. Lots of researchers have been able to make fusion happen, but so far the amount of energy taken to initiate the reaction has been more than we’ve gotten out of it so it has been a net loss of energy. Fusion would be amazing for a number of reasons (to be explained in upcoming posts), but so far, it’s not feasible. Check out Vancouver’s very own General Fusion for their great attempt at harnessing this highly coveted energy source.
How Nuclear Energy is Used
So, once the reactions are initiated, how do we use all the energy that comes out? Nuclear technology has a variety of applications, but since we’re talking about using it for energy, we’ll stick to those. In general, the energy released from nuclear reactors is converted to electricity for the electricity grid the reactors are attached to. The heat from the reaction is used to boil water which turns the water into high-pressure steam, which is then used to turn a turbine that creates electricity. Simple! This is very similar to what is done in several other power plants that run on fossil fuels. Below is a diagram showing the process:

The above diagram shows a reactor where lots of heat is released from the nuclear reaction. The heat is transferred to a fluid (often water) that is pumped to a containment vessel which creates high-pressure steam (steam generator). The high-pressure steam line connects to the turbine and powers it, and this turbine is connected to a generator that creates electricity. Afterwards, the still-quite-hot-but-lower-pressure steam runs by tubes of cool water (usually from a lake or reservoir) and is cooled off converting steam to water (this is the condensor). That water is pumped back up to turn into high-pressure steam again and the cycle keeps going. The water from the condensor is sent to a cooling tower to reject the excess heat into the atmosphere. This is a source of inefficiency as that heat could be used for something of value (and in some cases it is, e.g. heating buildings).
The heat from a nuclear reactor can be used directly by industrial processes as well (mining, manufacturing, chemical production, etc.), and is used to power the propulsion and operations of about 150 marine vessels worldwide. If you’ve ever heard the term “nuclear submarine”, now you know what it means.
OK! That’s all for now. Next post on energy will get into some specifics on the different types of nuclear fuels, both in practice today and promising experimental models.
Until then – hope you learned something about this particular energy source.
– G