I was intending to get into a series of posts on human energy needs, but figured it would be a better start to post on the basics of what energy is, what forms it takes, how it is measured, and a few other physical parameters involved. This will hopefully be a light, fun read, and will give readers a basic understanding of the vocabulary associated with energy. Here goes:
The Basics of Energy
Types of Energy
I touched upon this in my previous post, but I will take it further here. Energy is one thing, but comes in many forms. Some types of energy are easy to visualize, while other types require a theoretical understanding of what’s going on to understand how it’s used and measured. I will be focusing on types of energy that are relevant to the human use of energy as that is the focus of this here blog! Some types of energy are:
Kinetic Energy – this is quite simply the energy contained in things that are moving. When you walk, it is kinetic energy. When driving your car, the engine is putting out kinetic energy and transferring it to the car. Kinetic = movement. Simple!
Thermal Energy – this is heat. In some ways this is equivalent to kinetic energy (heat is moving molecules, after all), but when it comes to human energy consumption, it can be considered distinct. Hot things contain thermal energy! They transfer this energy to colder things.
Electrical Energy – this is energy stored in electric fields. Simply put, especially when considering human energy use, this is electricity. Zap! This is closely connected to magnetic energy, but isn’t as relevant to this discussion.
Radiant Energy – this is energy in the form of electromagnetic radiation. What’s easiest to visualize (literally) is light, which is a part of the electromagnetic spectrum. Read this post for more detail. In short – radio waves, microwaves, infrared rays, visible light, UV rays, X-rays, and gamma rays are all in this category.
Chemical Energy – this the energy stored in the bonds that create molecules from atoms. In gasoline, it’s just chilling there, until you burn the stuff and it gets released as heat, light, and kinetic energy.
Gravitational Energy – energy resulting from gravitational fields. Things really get moving when you drop them, don’t they?
Nuclear Energy – this is the energy stored in the nuclei of atoms. This is what keeps the nucleus together. Nuclear power plants use this to create electricity. And, um, bombs.
Elastic Energy – think about a spring. You push the spring away from where it sits when unstressed, and then *sproing*, it pops back. Elastic energy results from the deformation of objects.
Potential Energy – this is a bit more theoretical, and actually engulfs many of the above forms. Essentially, this is stored energy in any form. Chemical, gravitational, nuclear, and elastic are all examples of potential energy. Gravity provides the best example of this: if you have a swinging pendulum, as it rises up to one side, it slows down as the energy gets stored in the gravitational field, then it stops. Once it stops, it starts releasing the stored energy and starts moving down until it reaches its maximum speed at the lowest point. Check this animation:
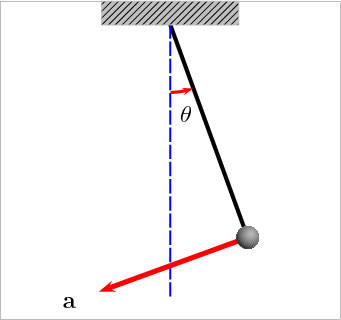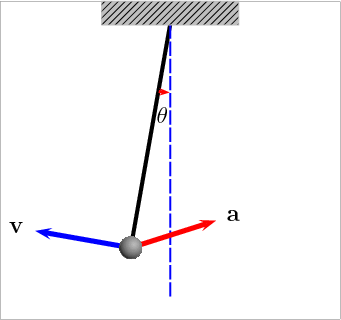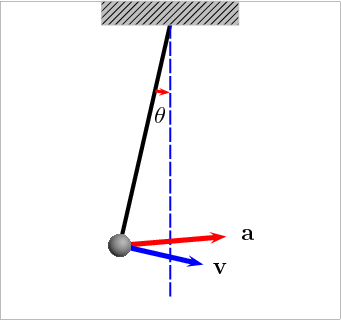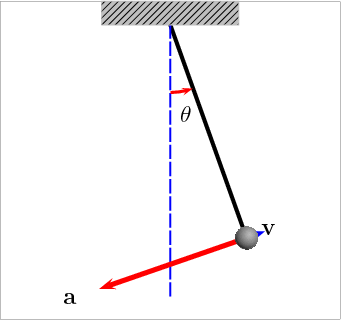
This list is not exhaustive, but I think you get the point so far. Energy is always the same thing in different forms. If you want to take it a little further, Albert Einstein even proved that energy and mass are the same thing in different forms. This is actually how nuclear power works – mass is converted to energy. We’ll stay away from that for now to keep things as simple as we can.
When looking at these different types of energy, the question arises (and has been hinted at above), “How do we get the type of energy that we want?”. Simple – convert one form to another. This can be done in many, many ways but every process that converts one form to another has to follow the golden rule: The Law of Conservation of Energy.
The Law of Conservation of Energy
Energy can neither be created or destroyed. Energy can only flow from one point to another, or from one form to another.
This is powerful. It means that energy is never “created” in that we can’t make new energy. All we can do is take it from one place to another, or from one form to another.
Examples:
Heat from a Campfire – Chemical energy stored in the bonds of the cellulose fibers (wood) are oxidized to create molecules that contain less chemical energy. The rest? Converted to light and heat. Many fond memories of this one…
Vehicle Movement – Let’s assume this is a gasoline powered vehicle. Chemical energy stored in the fuel is converted to heat, light, and movement in the form of expanding gases. This fires the pistons, which turns the wheels. Kinetic energy from fuel!
Solar Electricity – Electromagnetic radiation from the sun strikes a photovoltaic panel here on Earth, and is converted to electrical energy.
Hydro Electricity – Gravitational potential energy from water up high gets released as kinetic energy when the water drops down. That falling water can turn a turbine creating electricity.
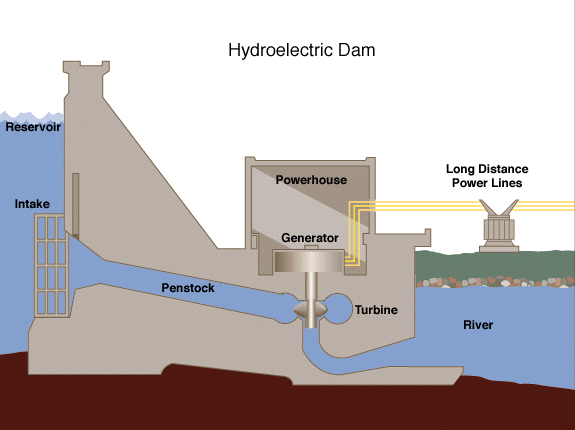
As one last tidbit of info, every time we convert energy from one form to another, some of it goes somewhere we don’t want, or converts to a form we don’t want, so these processes are never 100% efficient. This is usually called something akin to “energy loss”, but the energy isn’t lost, per se (that would violate the law of conservation); it just goes somewhere we can’t use it.
Energy vs Power
A lot of people use the words energy and power interchangeably, but in fact they are distinct from one another. Power, by definition, is the rate of transfer of energy. Let me say that again:
Power is the rate of transfer of energy. It is how much energy is transferred from one point to another, or one form to another, in a certain time.
Think of it this way: Moving a heavy object up the stairs requires a set amount of energy, whether you do it slowly or quickly. If you take three hours to get it up the stairs, you are not especially powerful. If you take 3 seconds to get it up the stairs, you are very powerful.
Another example: any car can get up to 100 km/hour, no problem (save some lame exceptions). Assuming that two different cars (a Porsche 911 Turbo and a Toyota Corolla, for example) are of near-equal mass, then the amount of energy required to get to 100 km/hour is almost the same. The Toyota Corolla takes about eight seconds to get up to 100 km/h, while the Porsche 911 Turbo can do it in three seconds. Which one do you think is more powerful? (Hint: it’s the Porsche).

Units of Energy and Power
So, how do we describe exactly how much energy something requires, or how quickly that energy needs to be transferred, i.e. power? We use units. There’s countless units for energy and power and they can be confusing. Here’s my attempt to clear it up:
There are two main unit systems in the world, one is SI (for ‘systeme internationale’, it’s French), aka the metric system, and the other is the imperial system. Non-energy units in the metric system include meters for length, kilograms for mass, and litres for volume. The imperial system uses things like inches and feet for length, pounds for mass (or weight), and gallons for volume.
Here are some examples of commonly used units in both systems for energy and power:
SI/Metric Units
Basic Unit for Energy – the Joule (J)
Basic Unit for Power – the Watt (W)
Remember, power is the amount of energy transferred per unit time. Due to this connection, there is a relationship between Joules and Watts. Here it is:
A Watt is 1 Joule transferred per second, or:
1 W = 1 J/s
Quite simple! Think of it this way: When you turn on a 60 W light bulb, it is turning 60 J of electrical energy into 60 J of light and heat every second. You might not want the heat, but hey, nothing’s perfect.
There’s another unit of energy that you might recognize from your electrical bill. It’s called the kilowatt-hour. It may seem strange that the word watt is in there, as that is usually reserved for power, not energy. Here’s the thing – since power is energy transferred per unit time, if we take a unit of power and multiply it by a unit of time, then we are back to energy again. So:
Kilowatt-hour (kWh) – A unit of energy that corresponds to a kilowatt (kilo means 1000, so 1000 W) of power being consumed for 1 hour. For you engineer/scientist types out there, it’s a quick exercise to show that 1 kWh is equivalent to 3,600,000 J.
There’s a few more SI/Metric ways to express energy and power, but they’re pretty obscure. Moving on!
Imperial Units
Before we get into the imperial units, let me preface this by saying that the imperial system is inherently confusing. There are many entries I could make here, but below are the ones I encounter the most. No worries if this section isn’t as clear. It’s not as clear to anyone…
Basic Unit for Energy: British Thermal Unit (BTU)
Basic Unit for Power 1: Horsepower (hp)
Basic Unit for Power 2: British Thermal Unit per Hour (BTU/hr)
To put them in terms of the SI system, A BTU is equivalent to about 1055 J, and a hp is equivalent to about 746 W.
You may notice that your furnace, boiler, or hot water heater has the unit BTU/hr. Since that is a unit of energy per unit of time, that is just another measurement for power that is often used in heating systems.

OK – That should do it for now! From my perspective, those are the basics of energy. Any questions?
